
Downloadcenter
Medical Device Regulation (MDR)
Sinds 26 mei 2021 gelden in de Europese Unie (EU) meer en strengere regels voor medische hulpmiddelen (MDR). Alle medische producten / hulpmiddelen dienen met een CE-markering te worden aangeduid. Met een CE-markering wordt aangegeven dat dit product voldoet aan de minimumeisen die de EU stelt op het gebied van veiligheid, gezondheid, milieu en consumentenbescherming. De CE-markering die op al onze producten van toepassing zijn tonen aan dat deze producten vrij verhandeld mogen worden binnen de EER - Europese Economische Ruimte. De producten met een CE-markering dienen volgens de nieuwe regelgeving middels een UDI (Unique Device Identification) te worden uitgegeven. Hieronder alle informatie over de intreding van de MDR.
What is the MDR?
The MDR (Medical Device Regulation) is the new European medical device regulation. It entered into force on May 25, 2017, and its date of application is mandatory as of May 26, 2021. The regulation forms the new EU legal framework for medical devices and replaces the previous Medical Device (MDD (93/42/EEC)).
The MDR defines extended obligations for all economic operators including manufacturers and distributors who are part of the supply chain of a medical device. Compliance with these requirements is a prerequisite for placing the medical devices on the Europe market.
In comparison to the MDD, the MDR as a European regulation does not have to be implemented into national law first. It will therefore apply directly in Germany from May 26, 2021.
What are the objectives of the MDR?
The MDR is intended to
- ensure a high level of health protection for patients and users
- set standards for increasing the quality and safety of medical devices
- guarantee a functioning EU internal market for medical devices
- introduce provisions to ensure transparency and traceability of medical devices throughout their life cycle
Implementation deadlines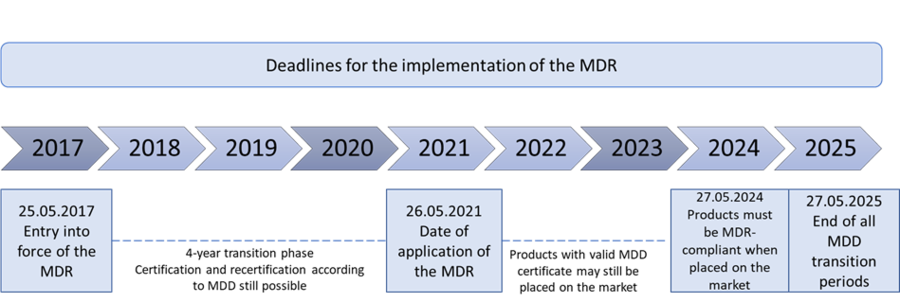
Important note:
All products placed on the market under the MDD by May 26, 2024 may be continued to be distributed until May 27, 2025 ("sell-off clause"). Distributors may sell MDD-compliant products that have already been placed on the market for the first time until May 27, 2025. Products that have already been supplied to end users (e.g. dental laboratories) by the end of this transitional period can be used after this date.
MDR: What's new?
- The MDR introduces the term "economic operartor" (which includes, among others,manufacturers and distributors and defines their obligations.
- The requirements for manufacturers with regard to the content of the Technical Documentation are significantly more extensive. Documents must be continuously updated.
- The obligations for post-market surveillance have been significantly expanded. This affects not only manufacturers, but also other[ economic operator.
- Clinical evaluations and clinical trials are regulated and required in more detail with regard to the form and quality of clinical data.
- The requirements for the information that must be supplied with a medical device (labeling, instructions for use) have been significantly expanded./li>
- Each product must be given a unique device identification number UDI.
- All medical devices must be labeled as medical devices. pritidenta will indicate this by using the "MD symbol". We print this symbol directly on the packaging.
- The classification of certain products will change.
- Medical device manufacturers must designate a qualified person in the company who must have extensive expertise in the field of medical devices and is responsible for regulatory compliance.
- The document retention period has been significantly extended from 5 to 10 (for implantable devices to 15) years.
- The requirements for Notified bodies have been increased. At the same time, they will in future be obliged to perform unannounced audits of manufacturers at least once every 5 years.
- The European database EUDAMED has been expanded. Up to now, only government institutions had access to EUDAMED. In the future, manufacturers, Notified Bodies and the public will also have access to it.
Hieronder vindt u een overzicht met links die verwijzen naar onze leveranciers, gebruiksinstructies, informatie- en veiligheidsbladen van onze MDR / CE-gecertificeerde producten.
D:
Dreve Dentamid_________________________________________
Dreve Sonic XL 4K Phrozen printer_____________________
E:
Exocad-DentalCAD Documentaion - index of Topics___
Exocad-Bar (steggen)module____________________________
P:
Pritidenta® GmbH_______________________________________
S:
Schütz Dental GmbH_____________________________________
Siladent GmbH___________________________________________
T:
Target 3D implant library________________________________
U:
unidesa-odi_______________________________________________
W:
Wassermann______________________________________________
![]() Adisil blauw - component A.pdf (175.8 KiB)
Adisil blauw - component A.pdf (175.8 KiB)
![]() Adisil blauw - component B - coloured.pdf (172.8 KiB)
Adisil blauw - component B - coloured.pdf (172.8 KiB)
![]() Adisil pink - component A.pdf (246.6 KiB)
Adisil pink - component A.pdf (246.6 KiB)
![]() Adisil pink - component B - coloured.pdf (246.0 KiB)
Adisil pink - component B - coloured.pdf (246.0 KiB)
![]() Adisil rapid - component A.pdf (169.9 KiB)
Adisil rapid - component A.pdf (169.9 KiB)
![]() Adisil rapid - component B.pdf (168.0 KiB)
Adisil rapid - component B.pdf (168.0 KiB)
![]() Adisil transparant - component A.pdf (177.0 KiB)
Adisil transparant - component A.pdf (177.0 KiB)
![]() Adisil transparant - component B.pdf (176.9 KiB)
Adisil transparant - component B.pdf (176.9 KiB)
![]() After_Bleaching_Care_(NL.pdf (39.9 KiB)
After_Bleaching_Care_(NL.pdf (39.9 KiB)
![]() Aktivierungsloesung_fuer_Vorvergoldung__(NL_).pdf (152.4 KiB)
Aktivierungsloesung_fuer_Vorvergoldung__(NL_).pdf (152.4 KiB)
![]() Alabastergips Spezial TYPE 2.pdf (148.7 KiB)
Alabastergips Spezial TYPE 2.pdf (148.7 KiB)
![]() Alabastergips Universal_1-5_gips.pdf (163.3 KiB)
Alabastergips Universal_1-5_gips.pdf (163.3 KiB)
![]() Algidur-Liquid.pdf (233.7 KiB)
Algidur-Liquid.pdf (233.7 KiB)
![]() Alginaat_Trealgin_Chromatic_NL.pdf (82.6 KiB)
Alginaat_Trealgin_Chromatic_NL.pdf (82.6 KiB)
![]() Alphablast_M_25_M_50_(NL.pdf (32.0 KiB)
Alphablast_M_25_M_50_(NL.pdf (32.0 KiB)
![]() AlphaDie_MF,_Fuellstoff__(NL_).pdf (31.9 KiB)
AlphaDie_MF,_Fuellstoff__(NL_).pdf (31.9 KiB)
![]() AlphaDie_MF,_Haerter__(NL_).pdf (162.5 KiB)
AlphaDie_MF,_Haerter__(NL_).pdf (162.5 KiB)
![]() AlphaDie_MF,_Harz_(NL.pdf (81.2 KiB)
AlphaDie_MF,_Harz_(NL.pdf (81.2 KiB)
![]() AlphaDie_separator_isolatiemiddel_NL.pdf (297.6 KiB)
AlphaDie_separator_isolatiemiddel_NL.pdf (297.6 KiB)
![]() Alphalink_Cem_NL.pdf (96.1 KiB)
Alphalink_Cem_NL.pdf (96.1 KiB)
![]() Alphalink_Implant_NL.pdf (39.4 KiB)
Alphalink_Implant_NL.pdf (39.4 KiB)
![]() Alphalot_Au-Cr_NL.pdf (32.4 KiB)
Alphalot_Au-Cr_NL.pdf (32.4 KiB)
![]() Alphalot_S_C_NL.pdf (32.8 KiB)
Alphalot_S_C_NL.pdf (32.8 KiB)
![]() Alphatop_RF_(NL.pdf (33.7 KiB)
Alphatop_RF_(NL.pdf (33.7 KiB)
![]() Aluminium Oxide.pdf (259.9 KiB)
Aluminium Oxide.pdf (259.9 KiB)
![]() Bims-Sep_puimsteenpasta.pdf (84.0 KiB)
Bims-Sep_puimsteenpasta.pdf (84.0 KiB)
![]() Bleach´n_Smile_Automix__GB_19052020.pdf (573.8 KiB)
Bleach´n_Smile_Automix__GB_19052020.pdf (573.8 KiB)
![]() CAM-Stone N TYPE 4.pdf (163.3 KiB)
CAM-Stone N TYPE 4.pdf (163.3 KiB)
![]() Capo_Bond_Aktivator_(NL.pdf (129.0 KiB)
Capo_Bond_Aktivator_(NL.pdf (129.0 KiB)
![]() Capo_Bulk_Fill_Capo_Bulk_Fill_Plus_DE.pdf (42.1 KiB)
Capo_Bulk_Fill_Capo_Bulk_Fill_Plus_DE.pdf (42.1 KiB)
![]() Capo_Capo_natural_Capo_Flow_(NL.pdf (75.4 KiB)
Capo_Capo_natural_Capo_Flow_(NL.pdf (75.4 KiB)
![]() Capo_Slow_Flow_(NL.pdf (75.1 KiB)
Capo_Slow_Flow_(NL.pdf (75.1 KiB)
![]() Detax_Freeprint® cast 2.0 UV_EN_1,01_983_n.pdf (141.2 KiB)
Detax_Freeprint® cast 2.0 UV_EN_1,01_983_n.pdf (141.2 KiB)
![]() Detax_Freeprint® cast UV_EN_2,04_11089_n.pdf (135.7 KiB)
Detax_Freeprint® cast UV_EN_2,04_11089_n.pdf (135.7 KiB)
![]() Detax_Freeprint® denture 385_EN_1,00_1115_n.pdf (154.2 KiB)
Detax_Freeprint® denture 385_EN_1,00_1115_n.pdf (154.2 KiB)
![]() Detax_Freeprint® gingiva UV_EN_1,00_1045_n.pdf (155.5 KiB)
Detax_Freeprint® gingiva UV_EN_1,00_1045_n.pdf (155.5 KiB)
![]() Detax_Freeprint® model 2.0 UV _EN_1,03_1062_n.pdf (161.6 KiB)
Detax_Freeprint® model 2.0 UV _EN_1,03_1062_n.pdf (161.6 KiB)
![]() Detax_Freeprint® model 385_EN_2,05_935_n.pdf (147.8 KiB)
Detax_Freeprint® model 385_EN_2,05_935_n.pdf (147.8 KiB)
![]() Detax_Freeprint® ortho 385_EN_1,06_839_n.pdf (154.0 KiB)
Detax_Freeprint® ortho 385_EN_1,06_839_n.pdf (154.0 KiB)
![]() Detax_Freeprint® ortho_EN_1,06_838_n.pdf (147.4 KiB)
Detax_Freeprint® ortho_EN_1,06_838_n.pdf (147.4 KiB)
![]() Detax_Freeprint® temp 385_EN_1,01_919_n.pdf (159.9 KiB)
Detax_Freeprint® temp 385_EN_1,01_919_n.pdf (159.9 KiB)
![]() Detax_Freeprint® tray UV_EN_1,02_925_n.pdf (147.7 KiB)
Detax_Freeprint® tray UV_EN_1,02_925_n.pdf (147.7 KiB)
![]() dialog_glaze_NL.pdf (33.8 KiB)
dialog_glaze_NL.pdf (33.8 KiB)
![]() dialog_Occlusal_NL.pdf (74.9 KiB)
dialog_Occlusal_NL.pdf (74.9 KiB)
![]() dialog_Opaker_poeder_Sebond_opaker_pink_NL.pdf (148.9 KiB)
dialog_Opaker_poeder_Sebond_opaker_pink_NL.pdf (148.9 KiB)
![]() dialog_Pastenopaker_(NL.pdf (96.8 KiB)
dialog_Pastenopaker_(NL.pdf (96.8 KiB)
![]() dialog_Polijstpasta_NL.pdf (31.5 KiB)
dialog_Polijstpasta_NL.pdf (31.5 KiB)
![]() dialog_Vario_(NL.pdf (74.2 KiB)
dialog_Vario_(NL.pdf (74.2 KiB)
![]() dialog_Vario_Chroma_Flow_NL.pdf (87.1 KiB)
dialog_Vario_Chroma_Flow_NL.pdf (87.1 KiB)
![]() dialog_Vario_Flow_(NL.pdf (75.0 KiB)
dialog_Vario_Flow_(NL.pdf (75.0 KiB)
![]() dialog_Vario_Pastaopaker_NL.pdf (95.1 KiB)
dialog_Vario_Pastaopaker_NL.pdf (95.1 KiB)
![]() dialog_Vario_Polish_NL.pdf (32.0 KiB)
dialog_Vario_Polish_NL.pdf (32.0 KiB)
![]() dialog™_bonding_fluid_DE.pdf (737.9 KiB)
dialog™_bonding_fluid_DE.pdf (737.9 KiB)
![]() dialog™_modelleervloeistof_GB.pdf (677.8 KiB)
dialog™_modelleervloeistof_GB.pdf (677.8 KiB)
![]() Diamant polijstpasta D7.pdf (350.1 KiB)
Diamant polijstpasta D7.pdf (350.1 KiB)
![]() Diamant polijstpasta D15.pdf (350.1 KiB)
Diamant polijstpasta D15.pdf (350.1 KiB)
![]() Die Keen natuurgips TYPE 5.pdf (163.3 KiB)
Die Keen natuurgips TYPE 5.pdf (163.3 KiB)
![]() Die Stone natuurgips TYPE 4.pdf (163.3 KiB)
Die Stone natuurgips TYPE 4.pdf (163.3 KiB)
![]() Dr. Balzer natuurgips TYPE 2.pdf (163.3 KiB)
Dr. Balzer natuurgips TYPE 2.pdf (163.3 KiB)
![]() Dreve_FotoDent_cast_385_405nm.pdf (138.8 KiB)
Dreve_FotoDent_cast_385_405nm.pdf (138.8 KiB)
![]() Dreve_FotoDent_dril guide.pdf (233.9 KiB)
Dreve_FotoDent_dril guide.pdf (233.9 KiB)
![]() Dreve_FotoDent_gingiva_385nm.pdf (91.1 KiB)
Dreve_FotoDent_gingiva_385nm.pdf (91.1 KiB)
![]() Dreve_Fotodent_IBT_385_nm.pdf (166.7 KiB)
Dreve_Fotodent_IBT_385_nm.pdf (166.7 KiB)
![]() Dreve_FotoDent_instructie_tray2.pdf (1.2 MiB)
Dreve_FotoDent_instructie_tray2.pdf (1.2 MiB)
![]() Dreve_FotoDent_model2_385nm.pdf (76.9 KiB)
Dreve_FotoDent_model2_385nm.pdf (76.9 KiB)
![]() Dreve_FotoDent_technische_data.pdf (36.9 KiB)
Dreve_FotoDent_technische_data.pdf (36.9 KiB)
![]() Dreve_Fotodent_tray_385_405nm.pdf (141.3 KiB)
Dreve_Fotodent_tray_385_405nm.pdf (141.3 KiB)
![]() EL-FORM_S_Galvano-Goldbad_(NL.pdf (38.2 KiB)
EL-FORM_S_Galvano-Goldbad_(NL.pdf (38.2 KiB)
![]() EL-FORM_S_Glanzzusatz_(NL.pdf (42.5 KiB)
EL-FORM_S_Glanzzusatz_(NL.pdf (42.5 KiB)
![]() EL-FORM_S_Vorvergoldungsbad_(NL.pdf (215.9 KiB)
EL-FORM_S_Vorvergoldungsbad_(NL.pdf (215.9 KiB)
![]() ELFORM_Galvano-Goldbad_(NL.pdf (38.2 KiB)
ELFORM_Galvano-Goldbad_(NL.pdf (38.2 KiB)
![]() ELFORM_Glanzzusatz_(NL.pdf (29.7 KiB)
ELFORM_Glanzzusatz_(NL.pdf (29.7 KiB)
![]() Excalibur natuurgips TYPE 4.pdf (163.3 KiB)
Excalibur natuurgips TYPE 4.pdf (163.3 KiB)
![]() Expansievloeistof Type 100.pdf (229.3 KiB)
Expansievloeistof Type 100.pdf (229.3 KiB)
![]() Flexor_CC_Glanzlack_(NL.pdf (30.1 KiB)
Flexor_CC_Glanzlack_(NL.pdf (30.1 KiB)
![]() Flexor_CC_Primer_(NL.pdf (174.1 KiB)
Flexor_CC_Primer_(NL.pdf (174.1 KiB)
![]() FotoDent_gingiva_385nm_Dreve.pdf (91.1 KiB)
FotoDent_gingiva_385nm_Dreve.pdf (91.1 KiB)
![]() FotoDent_model2_385nm_Dreve.pdf (76.9 KiB)
FotoDent_model2_385nm_Dreve.pdf (76.9 KiB)
![]() FuturAcryl_2000_poeder_NL.pdf (139.4 KiB)
FuturAcryl_2000_poeder_NL.pdf (139.4 KiB)
![]() FuturAcryl_2000_vloeistof_NL.pdf (288.9 KiB)
FuturAcryl_2000_vloeistof_NL.pdf (288.9 KiB)
![]() FuturaGen Pulver_GB_17102022.pdf (164.1 KiB)
FuturaGen Pulver_GB_17102022.pdf (164.1 KiB)
![]() FuturaGen vloeistof_NL_2018.pdf (289.1 KiB)
FuturaGen vloeistof_NL_2018.pdf (289.1 KiB)
![]() FuturaGen_LF_vloeistof_NL.pdf (292.5 KiB)
FuturaGen_LF_vloeistof_NL.pdf (292.5 KiB)
![]() Futura_Basic_Cold_poeder_NL.pdf (140.1 KiB)
Futura_Basic_Cold_poeder_NL.pdf (140.1 KiB)
![]() Futura_Basic_Cold_vloeistof_NL.pdf (289.1 KiB)
Futura_Basic_Cold_vloeistof_NL.pdf (289.1 KiB)
![]() Futura_Basic_Hot_poeder_NL.pdf (138.7 KiB)
Futura_Basic_Hot_poeder_NL.pdf (138.7 KiB)
![]() Futura_Basic_Hot_vloeistof_NL.pdf (288.9 KiB)
Futura_Basic_Hot_vloeistof_NL.pdf (288.9 KiB)
![]() Futura_Intensivfarben_(NL.pdf (33.8 KiB)
Futura_Intensivfarben_(NL.pdf (33.8 KiB)
![]() Futura_Jet_poeder_NL.pdf (139.3 KiB)
Futura_Jet_poeder_NL.pdf (139.3 KiB)
![]() Futura_Jet_vloeistof_NL.pdf (288.8 KiB)
Futura_Jet_vloeistof_NL.pdf (288.8 KiB)
![]() Futura_Polish_NL.pdf (136.8 KiB)
Futura_Polish_NL.pdf (136.8 KiB)
![]() Futura_Press_LT_poeder_NL.pdf (140.2 KiB)
Futura_Press_LT_poeder_NL.pdf (140.2 KiB)
![]() Futura_Press_LT_vloeistof_NL.pdf (288.6 KiB)
Futura_Press_LT_vloeistof_NL.pdf (288.6 KiB)
![]() Futura_Press_N_poeder_NL.pdf (139.4 KiB)
Futura_Press_N_poeder_NL.pdf (139.4 KiB)
![]() Futura_Press_N_vloeistof_NL.pdf (289.2 KiB)
Futura_Press_N_vloeistof_NL.pdf (289.2 KiB)
![]() Futura_Print_NL.pdf (136.7 KiB)
Futura_Print_NL.pdf (136.7 KiB)
![]() Futura_Self_poeder_NL.pdf (139.4 KiB)
Futura_Self_poeder_NL.pdf (139.4 KiB)
![]() Gipex_gipsoplosser.pdf (245.6 KiB)
Gipex_gipsoplosser.pdf (245.6 KiB)
![]() Gipsloeser_fuer_EL-FORM__(NL_).pdf (88.1 KiB)
Gipsloeser_fuer_EL-FORM__(NL_).pdf (88.1 KiB)
![]() Gips_oplosmiddel _NL.pdf (63.3 KiB)
Gips_oplosmiddel _NL.pdf (63.3 KiB)
![]() Glasstraalparels.pdf (200.2 KiB)
Glasstraalparels.pdf (200.2 KiB)
![]() Glasstraalparels_08102018.pdf (200.2 KiB)
Glasstraalparels_08102018.pdf (200.2 KiB)
![]() Hoogglans_polijstpasta_voor_Biotan_NL.pdf (113.1 KiB)
Hoogglans_polijstpasta_voor_Biotan_NL.pdf (113.1 KiB)
![]() HS_Cross_Liquid_(NL.pdf (179.6 KiB)
HS_Cross_Liquid_(NL.pdf (179.6 KiB)
![]() idofast_instructions_eng_unidesa_odi.pdf (117.8 KiB)
idofast_instructions_eng_unidesa_odi.pdf (117.8 KiB)
![]() Impla_Steri_Guide_(NL.pdf (109.5 KiB)
Impla_Steri_Guide_(NL.pdf (109.5 KiB)
![]() Kontursil - component A.pdf (173.2 KiB)
Kontursil - component A.pdf (173.2 KiB)
![]() Kontursil - component B.pdf (166.7 KiB)
Kontursil - component B.pdf (166.7 KiB)
![]() Marmodent® natuurgips TYPE 3.pdf (163.3 KiB)
Marmodent® natuurgips TYPE 3.pdf (163.3 KiB)
![]() Marmodent® S synthetisch gips TYPE 3.pdf (163.3 KiB)
Marmodent® S synthetisch gips TYPE 3.pdf (163.3 KiB)
![]() MarmoDie natuurgips TYPE 5.pdf (148.7 KiB)
MarmoDie natuurgips TYPE 5.pdf (148.7 KiB)
![]() Marmoplast® N superhardgips TYPE 4.pdf (163.3 KiB)
Marmoplast® N superhardgips TYPE 4.pdf (163.3 KiB)
![]() Marmorock® 20 natuurgips TYPE 4.pdf (163.3 KiB)
Marmorock® 20 natuurgips TYPE 4.pdf (163.3 KiB)
![]() Marmorock® 24 natuurgips TYPE 4.pdf (148.7 KiB)
Marmorock® 24 natuurgips TYPE 4.pdf (148.7 KiB)
![]() Marmorock® E natuurgips TYPE 5.pdf (163.3 KiB)
Marmorock® E natuurgips TYPE 5.pdf (163.3 KiB)
![]() Marmorock® Saphir synthetisch gips TYPE 4.pdf (163.3 KiB)
Marmorock® Saphir synthetisch gips TYPE 4.pdf (163.3 KiB)
![]() Marmorock® Speed natuurgips TYPE 4.pdf (163.3 KiB)
Marmorock® Speed natuurgips TYPE 4.pdf (163.3 KiB)
![]() MarmoScan-Spray Basic.pdf (371.0 KiB)
MarmoScan-Spray Basic.pdf (371.0 KiB)
![]() MarmoScan-Spray Plus.pdf (259.4 KiB)
MarmoScan-Spray Plus.pdf (259.4 KiB)
![]() Marmovest G_inbedmassa.pdf (156.5 KiB)
Marmovest G_inbedmassa.pdf (156.5 KiB)
![]() Microclean_reinigingsapparaat_eng.pdf (201.4 KiB)
Microclean_reinigingsapparaat_eng.pdf (201.4 KiB)
![]() Microform_gruen_Doubliermasse__(NL_).pdf (33.2 KiB)
Microform_gruen_Doubliermasse__(NL_).pdf (33.2 KiB)
![]() Microkorund_(NL.pdf (31.5 KiB)
Microkorund_(NL.pdf (31.5 KiB)
![]() Microlit_isi_NL.pdf (189.3 KiB)
Microlit_isi_NL.pdf (189.3 KiB)
![]() Micronium_Exclusiv_NL.pdf (184.8 KiB)
Micronium_Exclusiv_NL.pdf (184.8 KiB)
![]() Micronium_N_10_NL.pdf (184.4 KiB)
Micronium_N_10_NL.pdf (184.4 KiB)
![]() Micro_inbedmassa.pdf (213.1 KiB)
Micro_inbedmassa.pdf (213.1 KiB)
![]() Modelit® natuurgips TYPE 3.pdf (163.3 KiB)
Modelit® natuurgips TYPE 3.pdf (163.3 KiB)
![]() Natura natuurgips TYPE 3.pdf (163.3 KiB)
Natura natuurgips TYPE 3.pdf (163.3 KiB)
![]() Neo Marmorit® E modelhardgips TYPE 3.pdf (163.3 KiB)
Neo Marmorit® E modelhardgips TYPE 3.pdf (163.3 KiB)
![]() Neo Marmorit® natuurgips TYPE 3.pdf (163.3 KiB)
Neo Marmorit® natuurgips TYPE 3.pdf (163.3 KiB)
![]() Neo Marmorit® speed natuurgips TYPE 3.pdf (163.3 KiB)
Neo Marmorit® speed natuurgips TYPE 3.pdf (163.3 KiB)
![]() Neo Marmorit® super natuurgips.pdf (150.6 KiB)
Neo Marmorit® super natuurgips.pdf (150.6 KiB)
![]() Neo Marmorit® super natuurgips TYPE 3+4.pdf (163.3 KiB)
Neo Marmorit® super natuurgips TYPE 3+4.pdf (163.3 KiB)
![]() Neo Stone synthetisch gips TYPE 4.pdf (163.3 KiB)
Neo Stone synthetisch gips TYPE 4.pdf (163.3 KiB)
![]() Nuance_750_850_Glasurfluessigkeit_Malfarbenfluessigkeit__(NL_).pdf (33.1 KiB)
Nuance_750_850_Glasurfluessigkeit_Malfarbenfluessigkeit__(NL_).pdf (33.1 KiB)
![]() Nuance_750_850_Glasur_G_(NL.pdf (33.2 KiB)
Nuance_750_850_Glasur_G_(NL.pdf (33.2 KiB)
![]() Nuance_750_850_Glasur_Paste_Glace_(NL.pdf (32.7 KiB)
Nuance_750_850_Glasur_Paste_Glace_(NL.pdf (32.7 KiB)
![]() Nuance_750_850_Isolierfluessigkeit__(NL_).pdf (32.5 KiB)
Nuance_750_850_Isolierfluessigkeit__(NL_).pdf (32.5 KiB)
![]() Nuance_750_850_Keramikmassen_(NL.pdf (32.1 KiB)
Nuance_750_850_Keramikmassen_(NL.pdf (32.1 KiB)
![]() Nuance_750_850_Malfarben_(NL.pdf (31.4 KiB)
Nuance_750_850_Malfarben_(NL.pdf (31.4 KiB)
![]() Nuance_750_850_Modellierfluessigkeit_LM__(NL_).pdf (32.2 KiB)
Nuance_750_850_Modellierfluessigkeit_LM__(NL_).pdf (32.2 KiB)
![]() Nuance_750_850_Opakerfluessigkeit__(NL_).pdf (52.6 KiB)
Nuance_750_850_Opakerfluessigkeit__(NL_).pdf (52.6 KiB)
![]() Nuance_750_850_Pastenopaker_(NL.pdf (33.4 KiB)
Nuance_750_850_Pastenopaker_(NL.pdf (33.4 KiB)
![]() Occlusiespray_DFS_groen_DE.pdf (199.6 KiB)
Occlusiespray_DFS_groen_DE.pdf (199.6 KiB)
![]() Occlusiespray_Mark_S_groen_NL.pdf (239.7 KiB)
Occlusiespray_Mark_S_groen_NL.pdf (239.7 KiB)
![]() Orthoplaster natuurgips orthodontie.pdf (163.3 KiB)
Orthoplaster natuurgips orthodontie.pdf (163.3 KiB)
![]() Orthosin-Uni_vloeistof_NL.pdf (185.3 KiB)
Orthosin-Uni_vloeistof_NL.pdf (185.3 KiB)
![]() PaintAcryl_Fluessigkeit__(NL_).pdf (221.5 KiB)
PaintAcryl_Fluessigkeit__(NL_).pdf (221.5 KiB)
![]() PaintAcryl_Pulver_(NL.pdf (91.4 KiB)
PaintAcryl_Pulver_(NL.pdf (91.4 KiB)
![]() PCS-Cleaner_(NL.pdf (166.7 KiB)
PCS-Cleaner_(NL.pdf (166.7 KiB)
![]() PCS_Malfarbe_(NL.pdf (167.7 KiB)
PCS_Malfarbe_(NL.pdf (167.7 KiB)
![]() PCS_Primer_(NL.pdf (167.0 KiB)
PCS_Primer_(NL.pdf (167.0 KiB)
![]() Perawax gietwaspennen.pdf (209.7 KiB)
Perawax gietwaspennen.pdf (209.7 KiB)
![]() Poliresin_desinfecterend_polijstmiddel.pdf (428.4 KiB)
Poliresin_desinfecterend_polijstmiddel.pdf (428.4 KiB)
![]() Premium_inbedmassa.pdf (213.1 KiB)
Premium_inbedmassa.pdf (213.1 KiB)
![]() Premium_washechtmiddel.pdf (369.7 KiB)
Premium_washechtmiddel.pdf (369.7 KiB)
![]() Presto Vest II_inbedmassa.pdf (213.1 KiB)
Presto Vest II_inbedmassa.pdf (213.1 KiB)
![]() Pritidenta multidisc ZrO2.pdf (165.5 KiB)
Pritidenta multidisc ZrO2.pdf (165.5 KiB)
![]() Profisep Clean.pdf (420.4 KiB)
Profisep Clean.pdf (420.4 KiB)
![]() Reinigungsfluid_gruen__(saeurehaltig_)__(NL_).pdf (154.0 KiB)
Reinigungsfluid_gruen__(saeurehaltig_)__(NL_).pdf (154.0 KiB)
![]() Relight,_Haftvermittler_(NL.pdf (167.6 KiB)
Relight,_Haftvermittler_(NL.pdf (167.6 KiB)
![]() Relight,_Versiegelungslack_(NL.pdf (187.2 KiB)
Relight,_Versiegelungslack_(NL.pdf (187.2 KiB)
![]() Relight_Cleaner_(NL.pdf (166.4 KiB)
Relight_Cleaner_(NL.pdf (166.4 KiB)
![]() ReVeneer_Base_(NL.pdf (45.0 KiB)
ReVeneer_Base_(NL.pdf (45.0 KiB)
![]() ReVeneer_Composite_(NL.pdf (75.1 KiB)
ReVeneer_Composite_(NL.pdf (75.1 KiB)
![]() Sebond_Dentin_Bond_Opaker_(NL.pdf (88.9 KiB)
Sebond_Dentin_Bond_Opaker_(NL.pdf (88.9 KiB)
![]() Sebond_Grip_(NL.pdf (39.2 KiB)
Sebond_Grip_(NL.pdf (39.2 KiB)
![]() Sebond_Implant_(NL.pdf (129.1 KiB)
Sebond_Implant_(NL.pdf (129.1 KiB)
![]() Sebond_Opaker_Fluid_(NL.pdf (184.9 KiB)
Sebond_Opaker_Fluid_(NL.pdf (184.9 KiB)
![]() Sebond_Pink_(NL.pdf (92.9 KiB)
Sebond_Pink_(NL.pdf (92.9 KiB)
![]() Sebond_Smart_(NL.pdf (170.5 KiB)
Sebond_Smart_(NL.pdf (170.5 KiB)
![]() Sebond_Smart_Zirkonbonder_(NL.pdf (170.7 KiB)
Sebond_Smart_Zirkonbonder_(NL.pdf (170.7 KiB)
![]() Sebond_Universal_(NL.pdf (165.7 KiB)
Sebond_Universal_(NL.pdf (165.7 KiB)
![]() SeptProtectol_Plus_(NL.pdf (46.9 KiB)
SeptProtectol_Plus_(NL.pdf (46.9 KiB)
![]() Silaform Gingiva Soft.pdf (147.0 KiB)
Silaform Gingiva Soft.pdf (147.0 KiB)
![]() Silaform® 80 medium hard.pdf (200.2 KiB)
Silaform® 80 medium hard.pdf (200.2 KiB)
![]() Silaform® 85 K.pdf (194.5 KiB)
Silaform® 85 K.pdf (194.5 KiB)
![]() Silaform® 90 extra-hard.pdf (195.1 KiB)
Silaform® 90 extra-hard.pdf (195.1 KiB)
![]() Silaform® Gingiva.pdf (199.3 KiB)
Silaform® Gingiva.pdf (199.3 KiB)
![]() Silaform® Gingiva Sep.pdf (284.7 KiB)
Silaform® Gingiva Sep.pdf (284.7 KiB)
![]() Silaform® hardener.pdf (274.3 KiB)
Silaform® hardener.pdf (274.3 KiB)
![]() SilaPoly - blauw.pdf (340.1 KiB)
SilaPoly - blauw.pdf (340.1 KiB)
![]() SilaPoly - geel.pdf (340.3 KiB)
SilaPoly - geel.pdf (340.3 KiB)
![]() SilaPoly - groen.pdf (350.7 KiB)
SilaPoly - groen.pdf (350.7 KiB)
![]() SilaPoly - rood.pdf (340.2 KiB)
SilaPoly - rood.pdf (340.2 KiB)
![]() SilaPoly - wit.pdf (340.3 KiB)
SilaPoly - wit.pdf (340.3 KiB)
![]() SilaPoly - zwart.pdf (339.9 KiB)
SilaPoly - zwart.pdf (339.9 KiB)
![]() SilaPrint cast UV.pdf (288.5 KiB)
SilaPrint cast UV.pdf (288.5 KiB)
![]() SilaPrint model UV.pdf (195.5 KiB)
SilaPrint model UV.pdf (195.5 KiB)
![]() Silatray_102903.pdf (193.4 KiB)
Silatray_102903.pdf (193.4 KiB)
![]() Silvavest_GOLD_inbedmassa.pdf (213.1 KiB)
Silvavest_GOLD_inbedmassa.pdf (213.1 KiB)
![]() Sokkelgips FL vloeibaar TYPE 4.pdf (163.3 KiB)
Sokkelgips FL vloeibaar TYPE 4.pdf (163.3 KiB)
![]() Sokkelgips natuurgips TYPE 4.pdf (163.3 KiB)
Sokkelgips natuurgips TYPE 4.pdf (163.3 KiB)
![]() Straalmiddel HS_Strahlperlen.pdf (30.6 KiB)
Straalmiddel HS_Strahlperlen.pdf (30.6 KiB)
![]() TeleVest_inbedmassa.pdf (424.2 KiB)
TeleVest_inbedmassa.pdf (424.2 KiB)
![]() Temdent_Classic_poeder_NL.pdf (38.4 KiB)
Temdent_Classic_poeder_NL.pdf (38.4 KiB)
![]() Temdent_Classic_vloeistof_NL.pdf (287.3 KiB)
Temdent_Classic_vloeistof_NL.pdf (287.3 KiB)
![]() Titanium BioStar ° 2_4 .pdf (105.3 KiB)
Titanium BioStar ° 2_4 .pdf (105.3 KiB)
![]() Titanium BioStar °5.pdf (105.4 KiB)
Titanium BioStar °5.pdf (105.4 KiB)
![]() Tizian_Blank_5.0_ZrO2_ENG.pdf (103.6 KiB)
Tizian_Blank_5.0_ZrO2_ENG.pdf (103.6 KiB)
![]() Tizian_Blank_NEM_(NL.pdf (42.8 KiB)
Tizian_Blank_NEM_(NL.pdf (42.8 KiB)
![]() Tizian_Blank_PEEK_Tizian_Blank_PEEK_White_(NL.pdf (33.2 KiB)
Tizian_Blank_PEEK_Tizian_Blank_PEEK_White_(NL.pdf (33.2 KiB)
![]() Tizian_Blank_PMMA_(NL.pdf (32.5 KiB)
Tizian_Blank_PMMA_(NL.pdf (32.5 KiB)
![]() Tizian_Blank_PMMA_Splint_220220_220221_220222.pdf (806.5 KiB)
Tizian_Blank_PMMA_Splint_220220_220221_220222.pdf (806.5 KiB)
![]() Tizian_Blank_Polycarbonat_(NL.pdf (32.6 KiB)
Tizian_Blank_Polycarbonat_(NL.pdf (32.6 KiB)
![]() Tizian_Blank_Translucent_(NL.pdf (32.3 KiB)
Tizian_Blank_Translucent_(NL.pdf (32.3 KiB)
![]() Tizian_Fix_Basis_(NL.pdf (73.0 KiB)
Tizian_Fix_Basis_(NL.pdf (73.0 KiB)
![]() Tizian_Fix_Haerter__(NL_).pdf (267.6 KiB)
Tizian_Fix_Haerter__(NL_).pdf (267.6 KiB)
![]() Tizian_Flow_Resin_(NL.pdf (90.7 KiB)
Tizian_Flow_Resin_(NL.pdf (90.7 KiB)
![]() Tizian_Scanspray_(NL.pdf (137.2 KiB)
Tizian_Scanspray_(NL.pdf (137.2 KiB)
![]() Tizian_Ti_Keramikmassen_(NL.pdf (31.0 KiB)
Tizian_Ti_Keramikmassen_(NL.pdf (31.0 KiB)
![]() Tizian_Ti_Keramik_Glasurfluid_(NL.pdf (32.7 KiB)
Tizian_Ti_Keramik_Glasurfluid_(NL.pdf (32.7 KiB)
![]() Tizian_Ti_Keramik_Glasur_(NL.pdf (31.1 KiB)
Tizian_Ti_Keramik_Glasur_(NL.pdf (31.1 KiB)
![]() Tizian_Ti_Keramik_Liner_(NL.pdf (31.3 KiB)
Tizian_Ti_Keramik_Liner_(NL.pdf (31.3 KiB)
![]() Tizian_Ti_Keramik_Liner_Fluid_(NL.pdf (32.0 KiB)
Tizian_Ti_Keramik_Liner_Fluid_(NL.pdf (32.0 KiB)
![]() Tizian_Ti_Keramik_Malfarben_(NL.pdf (31.4 KiB)
Tizian_Ti_Keramik_Malfarben_(NL.pdf (31.4 KiB)
![]() Tizian_Ti_Keramik_Modellierfluid_(NL.pdf (31.9 KiB)
Tizian_Ti_Keramik_Modellierfluid_(NL.pdf (31.9 KiB)
![]() Tizian_Ti_Keramik_Opakerfluid_(NL.pdf (51.8 KiB)
Tizian_Ti_Keramik_Opakerfluid_(NL.pdf (51.8 KiB)
![]() Tizian_Ti_Keramik_Opaker_Pulver_(NL.pdf (31.5 KiB)
Tizian_Ti_Keramik_Opaker_Pulver_(NL.pdf (31.5 KiB)
![]() Tizian_Ti_Keramik_Schultermassenfluessigkeit__(NL_).pdf (32.3 KiB)
Tizian_Ti_Keramik_Schultermassenfluessigkeit__(NL_).pdf (32.3 KiB)
![]() Tizian_Ti_Keramik_Schultermassenisolierung_(NL.pdf (32.5 KiB)
Tizian_Ti_Keramik_Schultermassenisolierung_(NL.pdf (32.5 KiB)
![]() Tizian_Zirkonverstaerktes_Komposit__(NL_).pdf (32.6 KiB)
Tizian_Zirkonverstaerktes_Komposit__(NL_).pdf (32.6 KiB)
![]() TrayAcryl_86,_Fluessigkeit__(NL_).pdf (185.6 KiB)
TrayAcryl_86,_Fluessigkeit__(NL_).pdf (185.6 KiB)
![]() TrayAcryl_86,_Pulver_(NL.pdf (33.1 KiB)
TrayAcryl_86,_Pulver_(NL.pdf (33.1 KiB)
![]() Tresident_2000_DH_(NL.pdf (32.2 KiB)
Tresident_2000_DH_(NL.pdf (32.2 KiB)
![]() Ultrasoon_polijstpastareiniger.251020.pdf (232.3 KiB)
Ultrasoon_polijstpastareiniger.251020.pdf (232.3 KiB)
![]() Ultrasoon_reinigingsmiddel_voor_tandsteenafzetting.251011.pdf (239.4 KiB)
Ultrasoon_reinigingsmiddel_voor_tandsteenafzetting.251011.pdf (239.4 KiB)
